Background: Waldenström's macroglobulinaemia (WM) is an uncommon lymphoproliferative disorder. Although many options are available, an optimal first-line therapy for WM has not been defined. We postulated that combining bendamustine and rituximab (BR) with a next generation BTK inhibitor would result in deeper responses as measured by complete response (CR) and very good partial response (VGPR) rates, and provide a longer duration of response.
Objectives: The primary objective of this trial is to document the CR and VGPR rates
Methods: The BRAWM clinical trial combines BR with acalabrutinib in a one-year, fixed duration treatment course including six cycles of BR and 12 months of acalabrutinib (6 months of monotherapy). This ongoing trial is taking place at 9 clinical sites across Canada and 44 patients have been enrolled as of July 1, 2023, with a recruitment goal of 59.
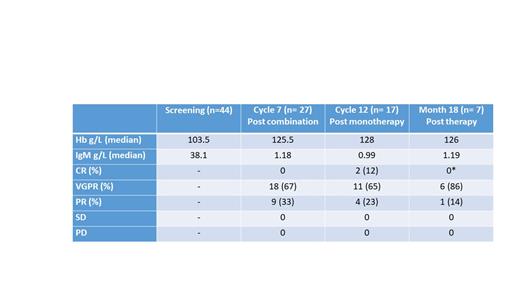
Results: Median age of participants was 68; 34 were male; 3 participants were low risk, 21 intermediate and 20 high risk. 27 participants completed combination therapy, 17 completed monotherapy and 7 were followed-up at 18 months, and 3 at 24 months after first dose. Table 1 shows the observed Clinical Responses using 6 th IWWM; 2 participants improved from a VGPR to a CR between cycles 7 and 12, both of whom have not reached month 18 for assessment. The interim primary endpoint of combined CR and VGPR rate is 13/17 (77%), with no participants having progressed at this interim analysis. 3 participants discontinued treatment early: 1 at cycle 7 (with a VGPR) who experienced an adverse event requiring treatment; 1 at cycle 3 with inter-current illness, and 1 at cycle 2 for personal reasons not related to treatment. The total observed Treatment Related Adverse Events (TRAEs) in combination therapy was 188 amongst 35 participants, and n= 24 in 10 participants during monotherapy. There were 24 Grade 3/4 TRAEs (combination n= 20, monotherapy n= 4). Total observed Serious Adverse Events was 18, and 7 TRSAEs 7; 6 during combination therapy (3 febrile neutropenia and 1 for each fever, allergic reaction and bowel obstruction) and 1 pneumonia during monotherapy. Grade 3/4 TRAEs resulted in 29 dose interruptions in 13 participants, and 2 dose reductions.
Mutational analysis of participants with adequate sample for assessment (n=35), showed 33 with MYD88 mutation, 10 with a CXCR4 mutation, and 1 with a TP53 mutation. Minimal residual disease (MRD) analysis of Peripheral Blood (PB) and Bone Marrow, using next generation sequencing, is underway. Initial review shows that most evaluable patients (those with samples at two or more time-points) have become MRD undetectable in PB. Uni- and multi-variate analyses of variables that may be associated with outcomes is underway.
Conclusions: Bendamustine, rituximab and acalabrutinib front-line therapy for WM is safe and well tolerated and initial clinical results show that this treatment induces a high percentage of CR + VGPRs.
Table 1: Clinical Responses using 6 th IWWM. *The 2 participants observed to have a CR at month 12 have not reached month 18 for progression or survival follow-up.
Disclosures
Berinstein:Astra Zeneca: Other: Scientific Advisor, Research Funding. Forward:AbbVie: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; InCyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; IMV: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; SeaGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; MorphoSys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees. Shafey:Roche: Honoraria; AstraZeneca: Honoraria. Nikonova:Forus: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Travel Grant; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; InCyte: Membership on an entity's Board of Directors or advisory committees; Apotex: Membership on an entity's Board of Directors or advisory committees. MacDonald:AbbVie: Honoraria; AstraZeneca: Honoraria; BeiGene: Honoraria; BMS: Honoraria; InCyte: Honoraria; Kite/Gilead: Honoraria; Roche: Honoraria; Seattle Genetics: Honoraria. Villa:Roche, AstraZeneca, Abbvie, Janssen, Kite/Gilead, BMS/Celgene, BeiGene, Merck: Consultancy, Honoraria; Roche, AstraZeneca: Research Funding. Sandhu:Janssen, Celgene/BMS, Gilead/Kite, Pfizer, Sanofi: Honoraria. Aljama:Pfizer, Sanofi and Beigene: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Gallucci:Adaptive Biotechnologies: Current Employment. Simmons:Adaptive Biotechnologies: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal